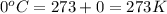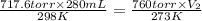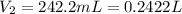Chemistry, 19.11.2019 03:31 carapiasebas
Atrial of this decomposition experiment, using different quantities of reactants than those listed in the question above produced the following data: volume of o2 produced at room conditions 280 ml barometric pressure 740 torr temperature of water 24°c termperature of o2 25°c vapor pressure due to water at 25°c 22.4 torr for the conditions listed above, calculate the volume of o2(g) produced at standard conditions of temperature and pressure. (enter your answer in liters)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
Atrial of this decomposition experiment, using different quantities of reactants than those listed i...
Questions


Computers and Technology, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10

Arts, 18.12.2020 21:10

Health, 18.12.2020 21:10

Chemistry, 18.12.2020 21:10


Mathematics, 18.12.2020 21:10


Mathematics, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10

Biology, 18.12.2020 21:10







Mathematics, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10

 produced at standard conditions of temperature and pressure is 0.2422 L
produced at standard conditions of temperature and pressure is 0.2422 L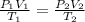
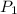 = initial pressure of
= initial pressure of 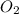 gas = (740-22.4) torr = 717.6 torr
gas = (740-22.4) torr = 717.6 torr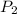 = final pressure of
= final pressure of 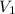 = initial volume of
= initial volume of 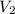 = final volume of
= final volume of 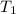 = initial temperature of
= initial temperature of 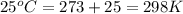
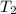 = final temperature of
= final temperature of