
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. chlorine trifluoride is prepared by the reaction cl2 (g) 3f2 (g) ⟶ 2clf3 (g). write the equation that relates the rate expressions for this reaction in terms of the disappearance of cl2 and f2 and the formation of clf3.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile co...
Questions

History, 14.07.2019 06:30

History, 14.07.2019 06:30



Social Studies, 14.07.2019 06:30

Chemistry, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30


Mathematics, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30


Mathematics, 14.07.2019 06:30


Mathematics, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30





Computers and Technology, 14.07.2019 06:30

![Rate=-\frac{d[Cl_2]}{dt}=-\frac{1}{3}\frac{d[F_2]}{dt}=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/d2692.png)
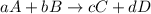
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0380/6825/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0380/6825/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0380/6825/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/d4b94.png)
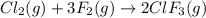
![\text{Rate of disappearance of }Cl_2=-\frac{d[Cl_2]}{dt}](/tpl/images/0380/6825/4403e.png)
![\text{Rate of disappearance of }F_2=-\frac{1}{3}\frac{d[F_2]}{dt}](/tpl/images/0380/6825/c24a1.png)
![\text{Rate of formation of }ClF_3=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/1400a.png)


