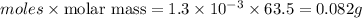Chemistry, 19.11.2019 05:31 nayellisoto15
Acopper cycle experiment takes copper atoms through reactions that produce copper compounds and complexes one after the other, finally producing elemental copper. copper atoms are conserved throughout the process. given that a student begins with 9.29 ml of a 0.14 m cu(no3)2 solution, how much copper should be isolated at the end of the cycle?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Acopper cycle experiment takes copper atoms through reactions that produce copper compounds and comp...
Questions






History, 23.08.2019 10:00

History, 23.08.2019 10:00



Mathematics, 23.08.2019 10:00

Mathematics, 23.08.2019 10:00


Health, 23.08.2019 10:00

Mathematics, 23.08.2019 10:00



History, 23.08.2019 10:00


English, 23.08.2019 10:00

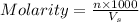
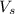 = volume of solution in ml = 9.29 ml
= volume of solution in ml = 9.29 ml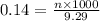
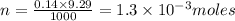
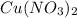 contains 1 mole of copper
contains 1 mole of copper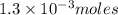 moles of
moles of 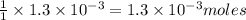 of copper
of copper