
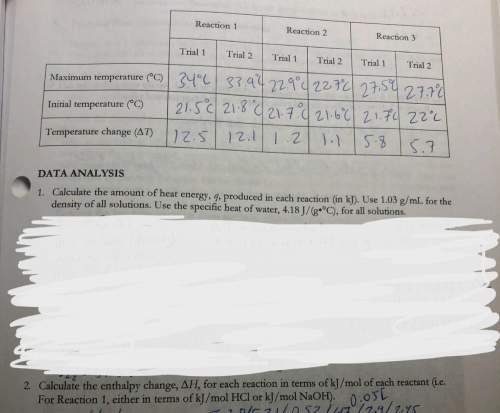
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for reaction three.
4. use hess’s law, and the accepted values of change of h in the pre-lab exercise to calculate the change in h for reaction 3. how does the accepted value compare to your experimental value?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1


Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for rea...
Questions



Mathematics, 20.11.2021 14:00




Chemistry, 20.11.2021 14:00

History, 20.11.2021 14:00


Mathematics, 20.11.2021 14:00


History, 20.11.2021 14:00

History, 20.11.2021 14:00


Mathematics, 20.11.2021 14:00


SAT, 20.11.2021 14:00



Mathematics, 20.11.2021 14:00



