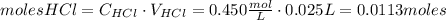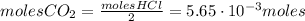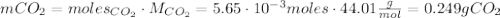Chemistry, 19.11.2019 06:31 caromaybelline71
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the following equation: caco3(s) 2hcl(aq)⟶co2(g) h2o(l) cacl2(aq) a typical antacid contains caco3. if such an antacid is added to 25.0 ml of a solution that is 0.450 m in hcl, how many grams of co2 gas are produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

You know the right answer?
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the followi...
Questions

Mathematics, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30


Chemistry, 08.10.2019 09:30



Biology, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30



Computers and Technology, 08.10.2019 09:30

History, 08.10.2019 09:30

Physics, 08.10.2019 09:30


Chemistry, 08.10.2019 09:30

Social Studies, 08.10.2019 09:30

Health, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30

Mathematics, 08.10.2019 09:30