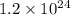Chemistry, 19.11.2019 09:31 patrickfryer240
Which of the following has 1.2 x 10^24 hydrogen atoms?
a) 1 mole of methane (ch4)
b) 2 moles of ammonia (nh 3)
c) 2 moles of hydrogen gas (h 2)
d) 1 mole of water (h 20)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Which of the following has 1.2 x 10^24 hydrogen atoms?
a) 1 mole of methane (ch4)
b) 2...
a) 1 mole of methane (ch4)
b) 2...
Questions

Mathematics, 16.12.2019 21:31



English, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31


Mathematics, 16.12.2019 21:31

Biology, 16.12.2019 21:31

Computers and Technology, 16.12.2019 21:31



History, 16.12.2019 21:31





Mathematics, 16.12.2019 21:31

Biology, 16.12.2019 21:31

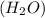
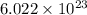 number of atoms.
number of atoms.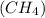
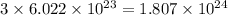 of hydrogen atoms.
of hydrogen atoms.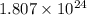
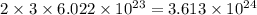 of hydrogen atoms.
of hydrogen atoms.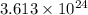
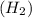
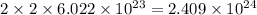 of hydrogen atoms.
of hydrogen atoms.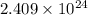
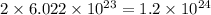 of hydrogen atoms.
of hydrogen atoms.