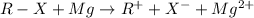Chemistry, 19.11.2019 17:31 pablogonzaleztellez
Arylmagnesium bromide is more stable than alkylmagnesium bromide. however, the reaction between aryl bromide and magnesium is slower than the reaction between alkyl bromide and the latter. explain this phenomenon.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
You know the right answer?
Arylmagnesium bromide is more stable than alkylmagnesium bromide. however, the reaction between aryl...
Questions



Arts, 18.05.2021 16:20


History, 18.05.2021 16:20



Mathematics, 18.05.2021 16:20

Biology, 18.05.2021 16:20

Mathematics, 18.05.2021 16:20

English, 18.05.2021 16:20

Mathematics, 18.05.2021 16:20


Mathematics, 18.05.2021 16:20

Mathematics, 18.05.2021 16:20

Mathematics, 18.05.2021 16:20



Mathematics, 18.05.2021 16:20