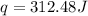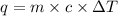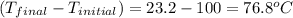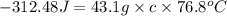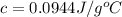You placed 43.1 g of an unknown metal at 100 °c into a coffee cup calorimeter that contained 50.0 g of water that was initially at 22.0 °c. the equilibrium temperature of mixing (t0) was determined to be 23.2 °c. the calorimeter constant was known to be 51.5 j/°c. specific heath2o = 4.184 j/g·°ca. what is the total amount of heat (j) lost by the metal? ng 1.5b. what was the specific heat (j/g·°c) of the metal? ng 1.5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 18:00
Astudent measured the ph for four solutions which ph indicated the lowest hydronium ion or concentration
Answers: 1

Chemistry, 23.06.2019 22:30
Which statement is true about this reaction? 14n+h–> 150 it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
You placed 43.1 g of an unknown metal at 100 °c into a coffee cup calorimeter that contained 50.0 g...
Questions

Mathematics, 14.01.2021 23:30





Biology, 14.01.2021 23:30

Social Studies, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30

History, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30

Health, 14.01.2021 23:30



English, 14.01.2021 23:30



Mathematics, 14.01.2021 23:30


Biology, 14.01.2021 23:30

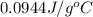
![q=[q_1+q_2]](/tpl/images/0381/4030/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0381/4030/1d50b.png)
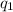 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter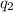 = heat absorbed by the water
= heat absorbed by the water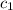 = specific heat of calorimeter =
= specific heat of calorimeter = 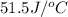
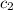 = specific heat of water =
= specific heat of water = 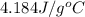
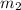 = mass of water = 50.0 g
= mass of water = 50.0 g = change in temperature =
= change in temperature = 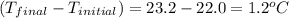
![q=[(51.5J/^oC\times 1.2^oC)+(50.0g\times 4.184J/g^oC\times 1.2^oC)]](/tpl/images/0381/4030/aca3d.png)