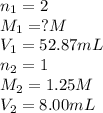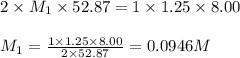Chemistry, 19.11.2019 17:31 zeesharpe05
8.00ml of 1.25m lithiukm hydroxide is reacted with sulfuric acid. it is found that 52.87ml of the sulfuric acid is required to completely neutralize the lithium hydroxide. what is the approximate molarity of sulfuric acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
8.00ml of 1.25m lithiukm hydroxide is reacted with sulfuric acid. it is found that 52.87ml of the su...
Questions






Mathematics, 11.12.2019 06:31












Computers and Technology, 11.12.2019 06:31


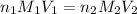
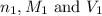 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 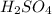
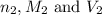 are the n-factor, molarity and volume of base which is LiOH
are the n-factor, molarity and volume of base which is LiOH