Chemistry, 19.11.2019 22:31 ronaldotheexplorer12
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj according to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj a) 1640 kj b) 1430 kj c) 429 kj d) 3070 kj e) 238 kj.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1


Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 a...
Questions




History, 20.03.2020 11:24

History, 20.03.2020 11:24



Mathematics, 20.03.2020 11:25







Mathematics, 20.03.2020 11:26

Mathematics, 20.03.2020 11:26




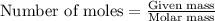 .....(1)
.....(1)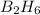 :
: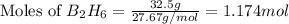
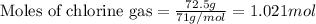
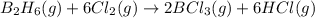
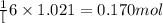 of
of