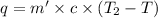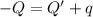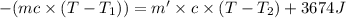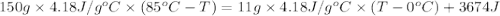Chemistry, 19.11.2019 22:31 Destinyb3722
Athermos contains 150 cm3 of coffee at 85 . to cool the coffee, you drop two 11-g ice cubes into the thermos. the ice cubes are initially at 0 and melt completely. what is the final temperature of the coffee? treat the coffee as if it were water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
Athermos contains 150 cm3 of coffee at 85 . to cool the coffee, you drop two 11-g ice cubes into the...
Questions

Mathematics, 23.04.2021 01:10


Mathematics, 23.04.2021 01:10

Chemistry, 23.04.2021 01:10

Mathematics, 23.04.2021 01:10

Mathematics, 23.04.2021 01:10

Mathematics, 23.04.2021 01:10


Biology, 23.04.2021 01:10


Mathematics, 23.04.2021 01:10

Mathematics, 23.04.2021 01:10



Mathematics, 23.04.2021 01:10


English, 23.04.2021 01:10

Mathematics, 23.04.2021 01:10


Mathematics, 23.04.2021 01:10

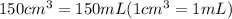
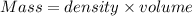
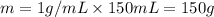
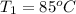
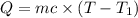
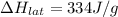

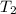 = 0°C
= 0°C