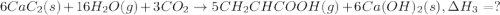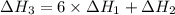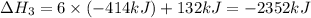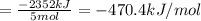Chemistry, 19.11.2019 23:31 sipstick9411
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s) (g) (g) (s) in the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions

Mathematics, 18.03.2021 02:10





Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10



English, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10


Biology, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Social Studies, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

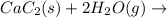
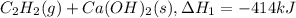 ..[1]
..[1]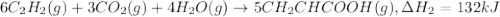 ..[2]
..[2]