

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Which of the following transitions represent the emission of a photon with the largest energy?
Questions

Computers and Technology, 16.11.2020 02:50

Mathematics, 16.11.2020 02:50


World Languages, 16.11.2020 02:50

Chemistry, 16.11.2020 02:50




English, 16.11.2020 02:50




Physics, 16.11.2020 02:50

Mathematics, 16.11.2020 02:50

Mathematics, 16.11.2020 02:50



Mathematics, 16.11.2020 02:50

History, 16.11.2020 02:50

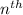 orbit is directly proportional to
orbit is directly proportional to 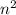 .Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to
.Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to  .So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).
.So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).


