
Chemistry, 20.11.2019 07:31 kittycat92
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid whose vapor pressure is the same as that of pure water at 20.0°c (18 torr). the volume of the mixture is 1.7 l, and its total pressure is 0.610 atm. determine the partial pressure of the hydrogen gas in this mixture.
a. 446 torr
b. 788 torr
c. 758 torr
d. 464 torr
e. 482 torr

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2



Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid who...
Questions

Mathematics, 01.07.2019 06:00

Mathematics, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00

Mathematics, 01.07.2019 06:00

Chemistry, 01.07.2019 06:00




English, 01.07.2019 06:00


Biology, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00


Mathematics, 01.07.2019 06:00

Biology, 01.07.2019 06:00

English, 01.07.2019 06:00

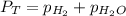
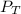 = total partial pressure = 0.610 atm = 463.6 torr
= total partial pressure = 0.610 atm = 463.6 torr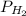 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?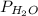 = partial pressure of water vapor = 18 torr
= partial pressure of water vapor = 18 torr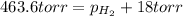
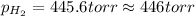
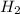 gas is, 446 torr
gas is, 446 torr

