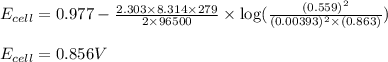Chemistry, 20.11.2019 17:31 hoosierkid5633
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (aq) + 2 h+ + 2 e- → 2 h2o eo = 1.78 v what will the potential of this cell be when [ag+] = 0.559 m, [h+] = 0.00393 m, and [h2o2] = 0.863 m?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (...
Questions





Mathematics, 24.06.2019 12:00




Health, 24.06.2019 12:00

Mathematics, 24.06.2019 12:00









Mathematics, 24.06.2019 12:00

Computers and Technology, 24.06.2019 12:00

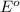 potential will always get reduced.
potential will always get reduced.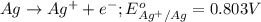 ( × 2)
( × 2)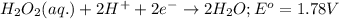
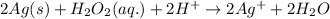
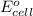 of the reaction, we use the equation:
of the reaction, we use the equation: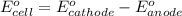
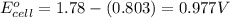
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ag^{+}]^2}{[H^{+}]^2[H_2O_2]}](/tpl/images/0383/1046/4a00d.png)
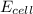 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Ag^{+}]=0.559M](/tpl/images/0383/1046/b53ec.png)
![[H^{+}]=0.00393M](/tpl/images/0383/1046/74117.png)
![[H_2O_2]=0.863M](/tpl/images/0383/1046/04f5b.png)