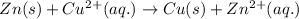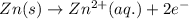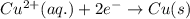Chemistry, 20.11.2019 17:31 fowers8376
Which species functions as the oxidizing agent in the following reduction-oxidation reaction:
zn(s) + cu^2+(aq) > cu(s) + zn^2+(aq)
a) zn^2+(aq)
b) zn(s)
c) cu^2+
d) cu(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
Which species functions as the oxidizing agent in the following reduction-oxidation reaction:
Questions

Mathematics, 27.01.2020 01:31






Mathematics, 27.01.2020 01:31

Mathematics, 27.01.2020 01:31


Mathematics, 27.01.2020 01:31


History, 27.01.2020 01:31



Mathematics, 27.01.2020 01:31




History, 27.01.2020 01:31

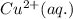 is the oxidizing agent for the given equation.
is the oxidizing agent for the given equation.