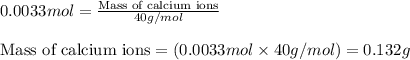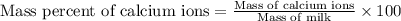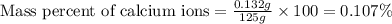The amount ofcalcium present in milk can be determined by adding oxalate to asample and measuring the massof calcium oxalate precipitated. what is the mass percent ofcalcium if 0.429 g of calcium oxalate forms in a125-g sample of milk when excess aqueous sodium oxalate isadded? na2c2o4(aq) +ca2+(aq) → cac2o4(s) +2na+(aq) a. 0.107% b. 0.202% c. 0.343% d. 1.10% e.1.37%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
The amount ofcalcium present in milk can be determined by adding oxalate to asample and measuring th...
Questions


Social Studies, 09.01.2020 17:31

Mathematics, 09.01.2020 17:31



Chemistry, 09.01.2020 17:31




Physics, 09.01.2020 17:31

Chemistry, 09.01.2020 17:31



Mathematics, 09.01.2020 17:31

Chemistry, 09.01.2020 17:31

Mathematics, 09.01.2020 17:31


Social Studies, 09.01.2020 17:31

Mathematics, 09.01.2020 17:31

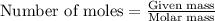 .....(1)
.....(1)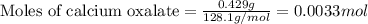
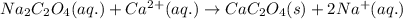
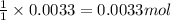 of calcium ions
of calcium ions