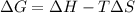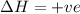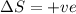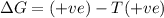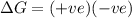Chemistry, 20.11.2019 20:31 Legoman29305
Consider a reaction that has a positive δh and a positive δs. which of the following statements is true? a) this reaction will be spontaneous only at high temperatures. b) this reaction will be spontaneous at all temperatures. c) this reaction will be nonspontaneous at all temperatures. d) this reaction will be nonspontaneous only at high temperatures. e) it is not possible to determine without more information.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
Consider a reaction that has a positive δh and a positive δs. which of the following statements is t...
Questions

Arts, 26.09.2019 14:10

Biology, 26.09.2019 14:10

Physics, 26.09.2019 14:10


English, 26.09.2019 14:10


Spanish, 26.09.2019 14:10



English, 26.09.2019 14:10


Health, 26.09.2019 14:10




English, 26.09.2019 14:10

Mathematics, 26.09.2019 14:10



Mathematics, 26.09.2019 14:10

 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous