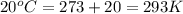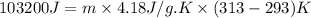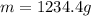Chemistry, 20.11.2019 20:31 bougiehairstudios
For many years drinking water has been cooled in hot climates by evaporating it from the surface of canvas bags or porous clay pots. how many grams of water can be cooled from 40 ∘c to 20 ∘c by the evaporation of 43 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj/g. the specific heat of water is 4.18 j/g⋅k.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
You know the right answer?
For many years drinking water has been cooled in hot climates by evaporating it from the surface of...
Questions


History, 19.01.2021 20:20

Mathematics, 19.01.2021 20:20



Health, 19.01.2021 20:20


Biology, 19.01.2021 20:20

Mathematics, 19.01.2021 20:20

Arts, 19.01.2021 20:20

Biology, 19.01.2021 20:20


Mathematics, 19.01.2021 20:20

Mathematics, 19.01.2021 20:20

Mathematics, 19.01.2021 20:20

Mathematics, 19.01.2021 20:20



Mathematics, 19.01.2021 20:20

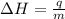
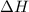 = enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g
= enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g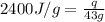
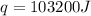
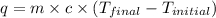
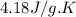
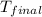 = final temperature =
= final temperature = 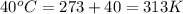
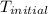 = initial temperature =
= initial temperature =