
Chemistry, 20.11.2019 21:31 smithsa10630
A40.0-ml solution contains 0.019 m barium chloride (bacl2). what is the minimum concentration of sodium sulfate (na2so4) required in the solution to produce a barium sulfate (baso4) precipitate? the solubility product for barium sulfate is ksp = 1.1 ✕ 10−10.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
A40.0-ml solution contains 0.019 m barium chloride (bacl2). what is the minimum concentration of sod...
Questions



Mathematics, 29.10.2020 03:30

English, 29.10.2020 03:30


Mathematics, 29.10.2020 03:30



Mathematics, 29.10.2020 03:30

English, 29.10.2020 03:30

English, 29.10.2020 03:30

Chemistry, 29.10.2020 03:30


Mathematics, 29.10.2020 03:30




Mathematics, 29.10.2020 03:30

Computers and Technology, 29.10.2020 03:30

History, 29.10.2020 03:30

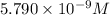 .
.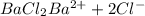
![[BaCl_2]=0.019 M](/tpl/images/0383/3321/1b1d1.png)
![[Ba^{2+}]](/tpl/images/0383/3321/77893.png)
![[Ba^{2+}]=[BaCl_2]=0.019 M](/tpl/images/0383/3321/edb0a.png)
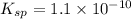
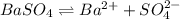
![K_{sp}=[Ba^{2+}]\times S](/tpl/images/0383/3321/9350a.png)
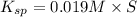
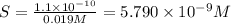
![[SO_4^{2-}]=5.790\times 10^{-9} M](/tpl/images/0383/3321/69505.png)
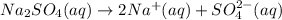
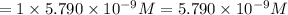 of sodium sulfate
of sodium sulfate

