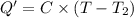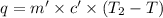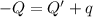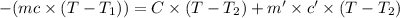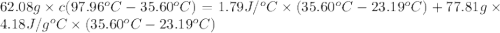In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 62.08 grams of magnesium to 97.96 °c and then drops it into a cup containing 77.81 grams of water at 23.19 °c. she measures the final temperature to be 35.60 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.79 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of magnesium.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1

Chemistry, 23.06.2019 13:30
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1
You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions

Biology, 16.10.2021 02:00

History, 16.10.2021 02:00


Mathematics, 16.10.2021 02:00

Biology, 16.10.2021 02:00


History, 16.10.2021 02:00

English, 16.10.2021 02:00


Advanced Placement (AP), 16.10.2021 02:00


SAT, 16.10.2021 02:00

Biology, 16.10.2021 02:00





English, 16.10.2021 02:10


Mathematics, 16.10.2021 02:10

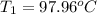
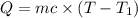
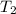 = 23.19°C
= 23.19°C