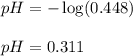Chemistry, 20.11.2019 23:31 infoneetusinghoyg22o
27.3 ml sample of a 0.488 m aqueous hydrofluoric acid solution is titrated with a 0.367 m aqueous potassium hydroxide solution. what is the ph at the start of the titration, before any potassium hydroxide has been added?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
27.3 ml sample of a 0.488 m aqueous hydrofluoric acid solution is titrated with a 0.367 m aqueous po...
Questions


Mathematics, 05.10.2019 02:00




Chemistry, 05.10.2019 02:00

Health, 05.10.2019 02:00

History, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00



Physics, 05.10.2019 02:00


History, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00

English, 05.10.2019 02:00


History, 05.10.2019 02:00

Social Studies, 05.10.2019 02:00

![pH=-\log[H^+]](/tpl/images/0383/5176/cf945.png)
![[H^+]=0.488M](/tpl/images/0383/5176/73a1e.png)