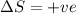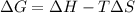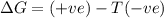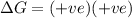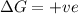Chemistry, 20.11.2019 23:31 annjetero2oy23ay
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably unfavorable; unfavorable; nonspontaneous unfavorable; favorable; spontaneous favorable; unfavorable; spontaneous favorable; unfavorable; nonspontaneous unfavorable; unfavorable; spontaneous

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction i...
Questions



Mathematics, 23.08.2019 16:00


English, 23.08.2019 16:00

History, 23.08.2019 16:00


Mathematics, 23.08.2019 16:00

Social Studies, 23.08.2019 16:00


Health, 23.08.2019 16:00


Mathematics, 23.08.2019 16:00



English, 23.08.2019 16:00


Mathematics, 23.08.2019 16:00

Mathematics, 23.08.2019 16:00

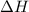 for Endothermic reaction is positive and
for Endothermic reaction is positive and 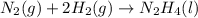
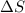 is negative as the randomness decreases when gases convert into liquid.
is negative as the randomness decreases when gases convert into liquid. 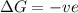 and favourable conditions are
and favourable conditions are 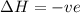 and
and