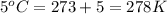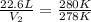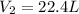Chemistry, 21.11.2019 01:31 laurencollett4838
An arctic weather balloon is filled with 22.6l of helium gas inside a prep shed. the temperature inside the shed is 7°c. the balloon is then taken outside, where the temperature is 5°c. calculate the new volume of the balloon.?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
An arctic weather balloon is filled with 22.6l of helium gas inside a prep shed. the temperature ins...
Questions

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01



Chemistry, 12.10.2020 19:01










Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

History, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

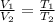
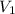 = initial volume of gas = 22.6 L
= initial volume of gas = 22.6 L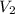 = final volume of gas = ?
= final volume of gas = ?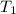 = initial temperature of gas =
= initial temperature of gas = 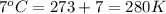
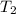 = final temperature of gas =
= final temperature of gas =