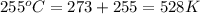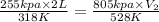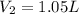Chemistry, 21.01.2020 02:31 dowlingdestiny
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases to 805 kilopascals as the temperature is raised to 255°c. what will be the new volume?
1.05 liters
2.28 liters
1.25 liters
0.44 liters
1.50 liters

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Agas at 255 kilopascals and 45°c has an initial volume of 2.00l. the pressure of the gas increases t...
Questions


English, 06.11.2020 17:50

Mathematics, 06.11.2020 17:50




English, 06.11.2020 17:50

Mathematics, 06.11.2020 17:50



Mathematics, 06.11.2020 17:50






Advanced Placement (AP), 06.11.2020 17:50



Mathematics, 06.11.2020 17:50

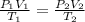
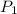 = initial pressure of gas = 255 kpa
= initial pressure of gas = 255 kpa = final pressure of gas = 805 kpa
= final pressure of gas = 805 kpa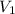 = initial volume of gas = 2 L
= initial volume of gas = 2 L
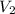 = final volume of gas = ?
= final volume of gas = ?
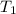 = initial temperature of gas =
= initial temperature of gas = 
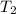 = final temperature of gas =
= final temperature of gas =