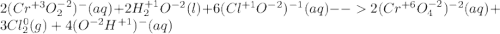Consider the following balanced redox reaction (do not include state of matter in your answers): 2cro2−(aq) + 2h2o(l) + 6clo−(aq) →2cro42−(aq) + 3cl2(g) + 4oh−(aq)(a) which species is being oxidized? (b) which species is being reduced? (c) which species is the oxidizing agent? (d) which species is the reducing agent? (e) from which species to which does electron transfer occur?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Consider the following balanced redox reaction (do not include state of matter in your answers): 2cr...
Questions












English, 23.09.2019 23:20




Mathematics, 23.09.2019 23:20


Business, 23.09.2019 23:20

Physics, 23.09.2019 23:20

Mathematics, 23.09.2019 23:20