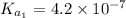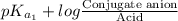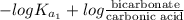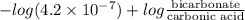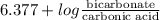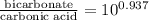The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonate]/[carbonic acid] = 0.11d) [bicarbonate]/[carbonic acid] = 9.4b) [bicarbonate]/[carbonic acid] = 0.38e) none of the above ratios is correct. c) [bicarbonate]/[carbonic acid] = 2.65

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions



Social Studies, 08.01.2020 23:31






Physics, 08.01.2020 23:31


English, 08.01.2020 23:31

Geography, 08.01.2020 23:31

Biology, 08.01.2020 23:31

Social Studies, 08.01.2020 23:31

Physics, 08.01.2020 23:31




History, 08.01.2020 23:31