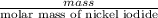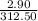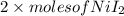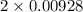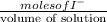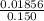Chemistry, 21.11.2019 03:31 maria051002camp
Uppose of nickel(ii) iodide is dissolved in of a aqueous solution of potassium carbonate. calculate the final molarity of iodide anion in the solution. you can assume the volume of the solution doesn't change when the nickel(ii) iodide is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

You know the right answer?
Uppose of nickel(ii) iodide is dissolved in of a aqueous solution of potassium carbonate. calculate...
Questions

World Languages, 13.10.2020 02:01



History, 13.10.2020 02:01


Chemistry, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Health, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01



Health, 13.10.2020 02:01


Biology, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01



English, 13.10.2020 02:01

Biology, 13.10.2020 02:01

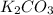 is added into the solution then it will not affect the
is added into the solution then it will not affect the 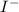 concentration.
concentration.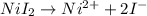
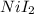 =
=