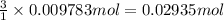The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with hydrogen: wo3(s)+3h2(g)→w(s)+3h2o(g)
part a how many grams of tungsten trioxide must you start with to prepare 1.80 g of tungsten? (for wo3, mw = 231.8 amu.)
part b how many grams of hydrogen must you start with to prepare 1.80 g of tungsten?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

You know the right answer?
The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with h...
Questions

Mathematics, 26.06.2019 22:00



Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00

History, 26.06.2019 22:00

English, 26.06.2019 22:00


Mathematics, 26.06.2019 22:00



Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00

Social Studies, 26.06.2019 22:00



Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00

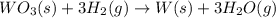
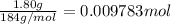
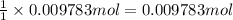 of tungsten trioxide
of tungsten trioxide