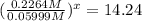At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same reaction’s rate constant was 7.42×10−3. it was noticed that when the reactant’s initial concentration was 0.2264 m (with a 593k reaction temperature), the initial reaction rate was identical to the initial rate when the decomposition was run at 673k with an initial reactant concentration of 0.05999 m. recall that rate laws have the form rate = k [a]x and, showing work, determine the order of the decomposition reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same rea...
Questions


Chemistry, 07.09.2021 01:20







Mathematics, 07.09.2021 01:20

Mathematics, 07.09.2021 01:20








Mathematics, 07.09.2021 01:20

Mathematics, 07.09.2021 01:20

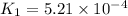
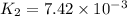
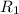
![R_1=K_1\times [A]^x](/tpl/images/0384/0720/5a42c.png)
![R_1=5.21\times 10^{-4}\times [A]^x](/tpl/images/0384/0720/5f894.png) ...[1]
...[1]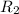
![R_2=K_2\times [A']^x](/tpl/images/0384/0720/6a78b.png)
![R_2=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/658af.png) ...[2]
...[2]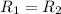 (given)
(given)![5.21\times 10^{-4}\times [A]^x=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/ebaac.png)
![(\frac{[A]}{[A']})^x=\frac{7.42\times 10^{-3}}{5.21\times 10^{-4}}](/tpl/images/0384/0720/a9fcc.png)