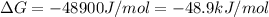Chemistry, 21.11.2019 04:31 alexreddin3127
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp (aq)+ h20 (l) -> adp (aq) + hpo4 (negative two overall charge) (aq).
for which ? g�rxn = �30.5 kj/mol at 37.0 �c and ph 7.0. calculate the value of ? grxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.80 mm, and [hpo42�] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions


History, 28.01.2021 01:00

English, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

German, 28.01.2021 01:00

Chemistry, 28.01.2021 01:00


Mathematics, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00


Mathematics, 28.01.2021 01:00

Biology, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

History, 28.01.2021 01:00

English, 28.01.2021 01:00

Mathematics, 28.01.2021 01:00

Physics, 28.01.2021 01:00


Mathematics, 28.01.2021 01:00

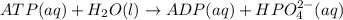
![[HPO_4^{2-}] = 5.0 mM=0.005 M](/tpl/images/0384/0544/f1ef4.png)
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}=\frac{0.0008 M\times 0.005 M}{0.005 M}=0.0008](/tpl/images/0384/0544/0fdb9.png)
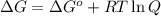
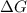 = Gibbs free energy at given conditions
= Gibbs free energy at given conditions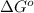 = Gibbs free energy at equilibrium=-30.5 kJ/mol
= Gibbs free energy at equilibrium=-30.5 kJ/mol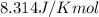
![37^oC=[273.15+37]K=310.15 K](/tpl/images/0384/0544/5ce7f.png)
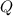 = reaction quotient at 37°C = 0.0008
= reaction quotient at 37°C = 0.0008![\Delta G=-30500 J/mol+(8.314J/Kmol)\times 310.15 K\times \ln [0.0008]](/tpl/images/0384/0544/d6332.png)