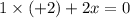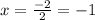Chemistry, 21.11.2019 04:31 rayniqueamee2002
Consider the lattice energies of the following group 2a compounds: beh2, 3205 kj/mol; mgh2, 2791 kj/mol; cah2, 2410 kj/mol; srh2, 2250 kj/mol; bah2, 2121 kj/mol. part a what is the oxidation number of h in these compounds?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Consider the lattice energies of the following group 2a compounds: beh2, 3205 kj/mol; mgh2, 2791 k...
Questions


English, 15.12.2021 08:20




Mathematics, 15.12.2021 08:20

Mathematics, 15.12.2021 08:20

Social Studies, 15.12.2021 08:20


Computers and Technology, 15.12.2021 08:20


Mathematics, 15.12.2021 08:20

English, 15.12.2021 08:20


Mathematics, 15.12.2021 08:20


Mathematics, 15.12.2021 08:20

Mathematics, 15.12.2021 08:20


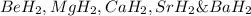 :
: