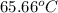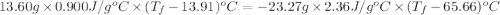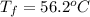Chemistry, 21.11.2019 05:31 tabocampos1414
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol with a temperature of 65.66 °c. when thermal equilibrium is reached, what is the temperature of the aluminum and ethylene glycol? specific heat capacities: lead = 0.159 j/g °c; ethylene glycol = 2.36 j/g ° °c

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol...
Questions





Biology, 24.02.2021 20:00


English, 24.02.2021 20:00




Mathematics, 24.02.2021 20:00




Mathematics, 24.02.2021 20:00

Physics, 24.02.2021 20:00




Mathematics, 24.02.2021 20:00

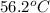
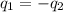
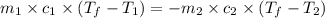
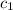 = specific heat of aluminum =
= specific heat of aluminum = 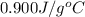
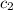 = specific heat of ethylene glycol =
= specific heat of ethylene glycol = 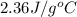
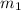 = mass of aluminum = 13.60 g
= mass of aluminum = 13.60 g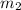 = mass of ethylene glycol = 23.27 g
= mass of ethylene glycol = 23.27 g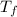 = final temperature of aluminum and ethylene glycol = ?
= final temperature of aluminum and ethylene glycol = ?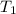 = initial temperature of aluminium =
= initial temperature of aluminium = 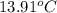
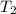 = initial temperature of ethylene glycol =
= initial temperature of ethylene glycol =