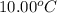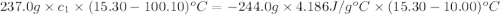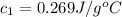A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup containing 244.0 g of water at 10.00 °c. if the final temperature of the water and metal in the cup is 15.30 °c, then what is the specific heat of molybdenum? (specific heat of water = 4.186 j/g-°c do not add the unit in the answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup co...
Questions

Biology, 28.08.2019 08:50



Mathematics, 28.08.2019 08:50




Mathematics, 28.08.2019 08:50



Physics, 28.08.2019 08:50

Social Studies, 28.08.2019 08:50

Mathematics, 28.08.2019 08:50


History, 28.08.2019 08:50

Social Studies, 28.08.2019 08:50


History, 28.08.2019 08:50


Mathematics, 28.08.2019 08:50

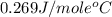
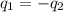
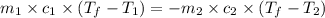
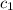 = specific heat of molybdenum metal = ?
= specific heat of molybdenum metal = ?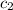 = specific heat of water =
= specific heat of water = 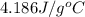
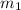 = mass of molybdenum metal = 237.0 g
= mass of molybdenum metal = 237.0 g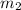 = mass of water = 244.0 g
= mass of water = 244.0 g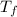 = final temperature of water and metal =
= final temperature of water and metal = 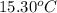
 = initial temperature of molybdenum metal =
= initial temperature of molybdenum metal = 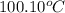
 = initial temperature of water =
= initial temperature of water =