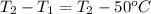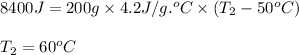Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

You know the right answer?
Ahot iron ball is dropped into a 200. g sample of water initially at 50°c. if 8.4 kj of heat is tran...
Questions





History, 20.09.2019 08:30


Mathematics, 20.09.2019 08:30

History, 20.09.2019 08:30


Biology, 20.09.2019 08:30

Chemistry, 20.09.2019 08:30

Biology, 20.09.2019 08:30








History, 20.09.2019 08:30

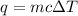
 = change in temperature =
= change in temperature =