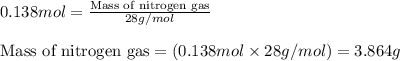Chemistry, 21.11.2019 20:31 rileyeddins1010
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to inflate airbags: 2 nan3 (s) 2 na (s) + 3 n2 (g) how many grams of n2 are produced from 6.00 g of nan

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to infla...
Questions

Business, 05.05.2020 13:24

English, 05.05.2020 13:24



Social Studies, 05.05.2020 13:24

Chemistry, 05.05.2020 13:24

Mathematics, 05.05.2020 13:24


Business, 05.05.2020 13:24

Biology, 05.05.2020 13:24

English, 05.05.2020 13:24


Chemistry, 05.05.2020 13:24

Mathematics, 05.05.2020 13:24

English, 05.05.2020 13:24



English, 05.05.2020 13:24


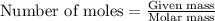 .....(1)
.....(1)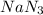 = 6.00 g
= 6.00 g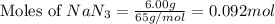
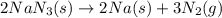
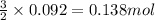 of nitrogen gas
of nitrogen gas