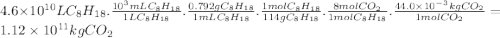Chemistry, 21.11.2019 20:31 jacksonyodell4184
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that the density of isooctane is 0.792 g/ml, what mass of co2 (in kilograms) is produced each year by the annual u. s. gasoline consumption of 4.6×1010l?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1


You know the right answer?
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that...
Questions


Chemistry, 27.10.2020 01:30

Mathematics, 27.10.2020 01:30



Geography, 27.10.2020 01:30

Mathematics, 27.10.2020 01:30


Mathematics, 27.10.2020 01:30








Mathematics, 27.10.2020 01:30

Mathematics, 27.10.2020 01:30

Chemistry, 27.10.2020 01:30