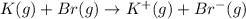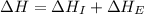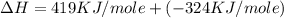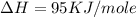Chemistry, 21.11.2019 21:31 shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions


Mathematics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31

Mathematics, 15.01.2020 01:31




Mathematics, 15.01.2020 01:31



Chemistry, 15.01.2020 01:31

History, 15.01.2020 01:31

English, 15.01.2020 01:31






Mathematics, 15.01.2020 01:31

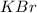 :
: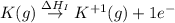
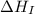 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol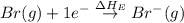
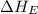 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol