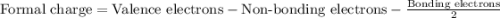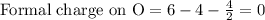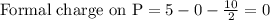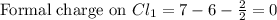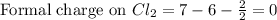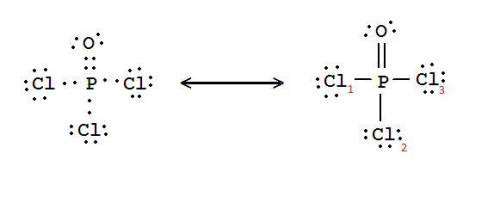Phosphorus forms a number of oxohalides, x3po, in which x may be a f, cl, or br atom. the most common of these, phosphoryl chloride, is obtained through the reaction 2pcl3(g)+o2(g)⟶2cl3po(g) draw the lewis structure for phosphoryl chloride. optimize formal charges.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Phosphorus forms a number of oxohalides, x3po, in which x may be a f, cl, or br atom. the most commo...
Questions

English, 29.11.2021 22:00


History, 29.11.2021 22:00

Mathematics, 29.11.2021 22:00


Law, 29.11.2021 22:00


Mathematics, 29.11.2021 22:00





Mathematics, 29.11.2021 22:00




Mathematics, 29.11.2021 22:00



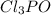 is shown below.
is shown below.