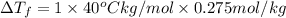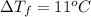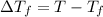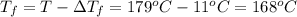Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Assume the molality of isoborneol in your product is 0.275 mol/kg. what is the melting point of your...
Questions

Mathematics, 14.12.2021 06:10

Mathematics, 14.12.2021 06:10


Mathematics, 14.12.2021 06:10

Mathematics, 14.12.2021 06:10

History, 14.12.2021 06:10



Mathematics, 14.12.2021 06:10

Mathematics, 14.12.2021 06:10

Computers and Technology, 14.12.2021 06:10


Mathematics, 14.12.2021 06:10




Mathematics, 14.12.2021 06:10

Mathematics, 14.12.2021 06:20

Chemistry, 14.12.2021 06:20

Mathematics, 14.12.2021 06:20

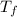 = ?
= ?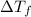
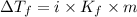
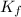 = The freezing point depression constant
= The freezing point depression constant