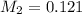Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
If 0.290g of fas is dissolved in 10ml of di water and is titrated to equivalence with 12.23ml kmno4,...
Questions

History, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01


Spanish, 04.10.2020 14:01

History, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01

History, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01




History, 04.10.2020 14:01

Computers and Technology, 04.10.2020 14:01


English, 04.10.2020 14:01

Physics, 04.10.2020 14:01

History, 04.10.2020 14:01

Chemistry, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01

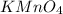 is 0.121 M
is 0.121 M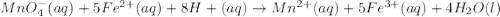
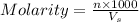
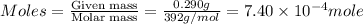
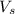 = volume of solution = 10 ml
= volume of solution = 10 ml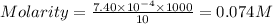
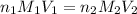
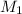 = molarity of
= molarity of 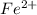 solution = 0.074 M
solution = 0.074 M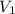 = volume of
= volume of 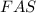 solution = 10 ml
solution = 10 ml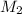 = molarity of
= molarity of 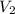 = volume of
= volume of 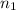 = valency of
= valency of 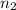 = valency of
= valency of