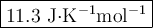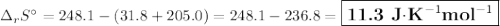The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) → so2 (g) is j/k⋅mol. the value of s° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) so2 (g) is j/kmol. +248.5 +485.4 +11.6 -11.6 -248.5

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombi...
Questions

Physics, 04.12.2019 19:31






Mathematics, 04.12.2019 19:31

Mathematics, 04.12.2019 19:31



Biology, 04.12.2019 19:31


English, 04.12.2019 19:31

Social Studies, 04.12.2019 19:31


Mathematics, 04.12.2019 19:31



English, 04.12.2019 19:31