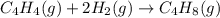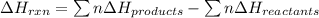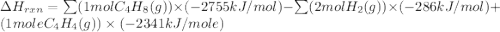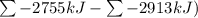Chemistry, 22.11.2019 00:31 montrellgoodman5890
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. using the enthalpies of combustion for c4h4 (-2341 kj/mol), c4h8 (-2755 kj/mol), and h2 (-286 kj/mol), calculate δh for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and h...
Questions

Mathematics, 21.11.2019 19:31

Mathematics, 21.11.2019 19:31

Mathematics, 21.11.2019 19:31


Geography, 21.11.2019 19:31

Mathematics, 21.11.2019 19:31

Mathematics, 21.11.2019 19:31





Health, 21.11.2019 19:31

English, 21.11.2019 19:31





Mathematics, 21.11.2019 19:31

Social Studies, 21.11.2019 19:31