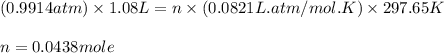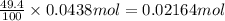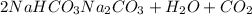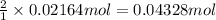You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of nahco3 (s) are required to fill this container given a 49.4% co2 recovery, assuming the other contents in the bag take up a negligible volume compared to the gas. the temperature of the room is 24.5 °c and the atmospheric pressure is 753.5 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of...
Questions






Physics, 20.03.2020 01:31








English, 20.03.2020 01:31

Mathematics, 20.03.2020 01:31

Chemistry, 20.03.2020 01:32



English, 20.03.2020 01:32

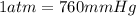 )
)