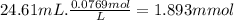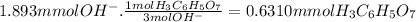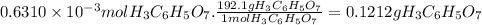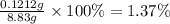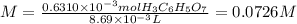Chemistry, 22.11.2019 00:31 eaglesjohnson414
Consider a different analyte for this exercise. citric acid is found in many fruits and fruit juices. sodium hydroxide (naoh) is the titrant and citric acid (h3c6h5o7) the analyte according to the following balanced chemical equation. h3c6h5o7 + 3 oh − → c6h5o73− + 3 h2o
(a) what is the stoichiometry of h3c6h5o7 to oh −?
(b) complete the following table for this titration. data table p2: titration of citric acid in orange juice with sodium hydroxide.
concentration of oh − 0.0769 m
volume orange juice 8.69 ml
mass orange juice 8.83 g
volume of oh − solution 24.61 ml
questions:
mmol of oh −: ?
mmol of h3c6h5o7: ?
mass of h3c6h5o7: ?
mass % of h3c6h5o7 in orange juice: ?
molarity of h3c6h5o7 in orange juice: ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
Consider a different analyte for this exercise. citric acid is found in many fruits and fruit juices...
Questions

Biology, 10.06.2021 15:10



Mathematics, 10.06.2021 15:10


Mathematics, 10.06.2021 15:10



Health, 10.06.2021 15:10

Mathematics, 10.06.2021 15:10

Business, 10.06.2021 15:10

Mathematics, 10.06.2021 15:10

Mathematics, 10.06.2021 15:10

Mathematics, 10.06.2021 15:10

Computers and Technology, 10.06.2021 15:10

Mathematics, 10.06.2021 15:10


Mathematics, 10.06.2021 15:10