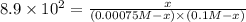Chemistry, 22.11.2019 01:31 ellenaschool
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through the formation of the red fe(h2o)5scn2+ (or, more simply, fescn2+). what is [fe3+] when 0.700 l each of 0.00150 m fe(no3)3 and 0.200 m kscn are mixed? kf of fescn2+ = 8.9 × 102. enter your answer in scientific notation. report your final answer to two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through t...
Questions





Chemistry, 08.07.2019 02:10

Mathematics, 08.07.2019 02:10




Mathematics, 08.07.2019 02:10



Mathematics, 08.07.2019 02:10

Mathematics, 08.07.2019 02:10

Social Studies, 08.07.2019 02:10


Mathematics, 08.07.2019 02:10


Mathematics, 08.07.2019 02:10

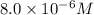 .
.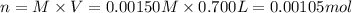
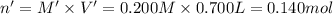
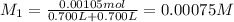
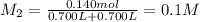
![Fe^{3+}+SCN^-\rightleftharpoons [Fe(SCN)]^{2+}](/tpl/images/0385/5120/942bc.png)
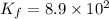
![K_f=\frac{[[Fe(SCN)]^{2+}]}{[[Fe^{3+}]][SCN^{-}]}](/tpl/images/0385/5120/f34ae.png)