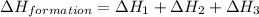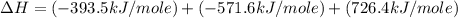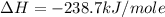Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 --> co2(g) latex: \deltaδh° = –393.5 kj/mol h2(g) + (1/2)o2 --> h2o(l) latex: \deltaδh° = –285.8 kj/mol ch3oh(l) + (3/2)o2(g) --> co2(g) + 2h2o(l) latex: \deltaδh° = –726.4 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following infor...
Questions



Chemistry, 10.02.2021 05:30


Mathematics, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

Social Studies, 10.02.2021 05:30


Social Studies, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30


Biology, 10.02.2021 05:30

Chemistry, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

Mathematics, 10.02.2021 05:30

English, 10.02.2021 05:30

Business, 10.02.2021 05:30

 will be,
will be,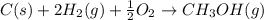
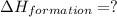
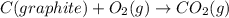
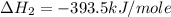
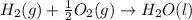
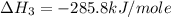
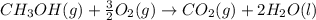
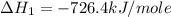
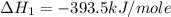
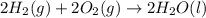
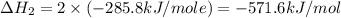
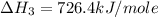
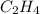 will be,
will be,