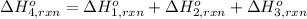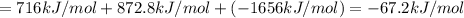Chemistry, 22.11.2019 03:31 CyberSongWriter
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estimate the standard enthalpy of formation of methane (ch4). c(s) → c(g) δh o rxn = 716 kj/mol 2h2(g) → 4h(g) δh o rxn = 872.8 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estim...
Questions


Social Studies, 12.07.2019 15:30


Biology, 12.07.2019 15:30

History, 12.07.2019 15:30

Biology, 12.07.2019 15:30

Biology, 12.07.2019 15:30

Mathematics, 12.07.2019 15:30

Mathematics, 12.07.2019 15:30


Mathematics, 12.07.2019 15:30

Mathematics, 12.07.2019 15:30




Biology, 12.07.2019 15:30


Biology, 12.07.2019 15:30

Mathematics, 12.07.2019 15:30

Biology, 12.07.2019 15:30

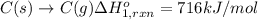 ...[1]
...[1]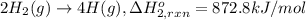 ...[2]
...[2]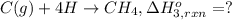 ...[3]
...[3]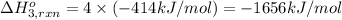
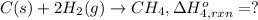 ...[4]
...[4]