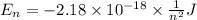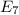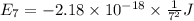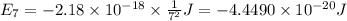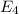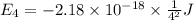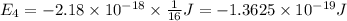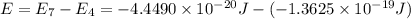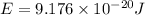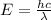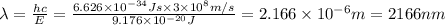Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Calculate the wavelength, in nanometers, of the light emitted by a hydrogen atom when its electron f...
Questions



Chemistry, 29.07.2019 03:30

Social Studies, 29.07.2019 03:30



English, 29.07.2019 03:30



Mathematics, 29.07.2019 03:30

Physics, 29.07.2019 03:30



Social Studies, 29.07.2019 03:30


Mathematics, 29.07.2019 03:30

Mathematics, 29.07.2019 03:30


Biology, 29.07.2019 03:30

History, 29.07.2019 03:30