Chemistry, 22.11.2019 04:31 wutwut2261
The absorbance of a crystal violet solution in water (density is 1.00 g/ml) is measured in a 1.0 cm cuvette. the absorbance is 0.614 and the molar absorptivity coefficient is the molecular weight of crystal violet is 407.98 g/mol. what is the molar concentration of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1


Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
The absorbance of a crystal violet solution in water (density is 1.00 g/ml) is measured in a 1.0 cm...
Questions


Computers and Technology, 17.10.2019 06:10

Computers and Technology, 17.10.2019 06:10

Mathematics, 17.10.2019 06:10





Geography, 17.10.2019 06:20

History, 17.10.2019 06:20




Mathematics, 17.10.2019 06:20

History, 17.10.2019 06:20


Social Studies, 17.10.2019 06:20



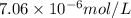

 = molar absorptivity coefficient =
= molar absorptivity coefficient = 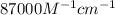 (assume)
(assume)